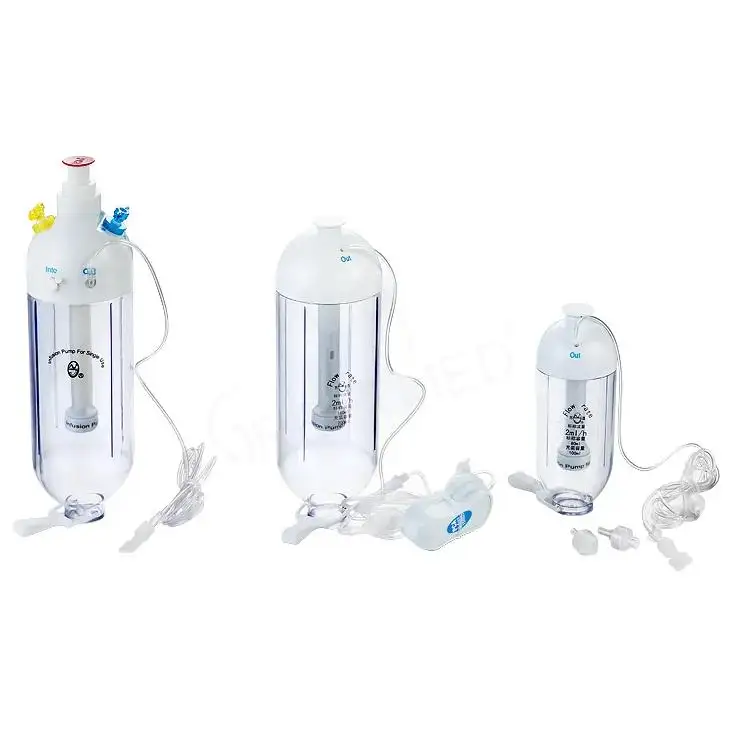
Top Criteria for Selecting an IV Sets and Cannulas Supplier You Trust
Selecting an IV sets and cannulas supplier is not a simple box-checking exercise. The choice influences dosing precision at the bedside, how often clinicians have to troubleshoot small failures, and how resilient your hospital or distribution network stays when supply pressures hit. The wrong partner brings drift in quality, gaps during audits, and shortages that push risky substitutions. The right partner delivers consistent performance, clearer documentation, and smoother clinical routines. This guide highlights the criteria that truly matter, the pitfalls to avoid, and how Greetmed translates rigorous engineering and quality discipline into day-to-day value for hospitals, distributors, and frontline teams.
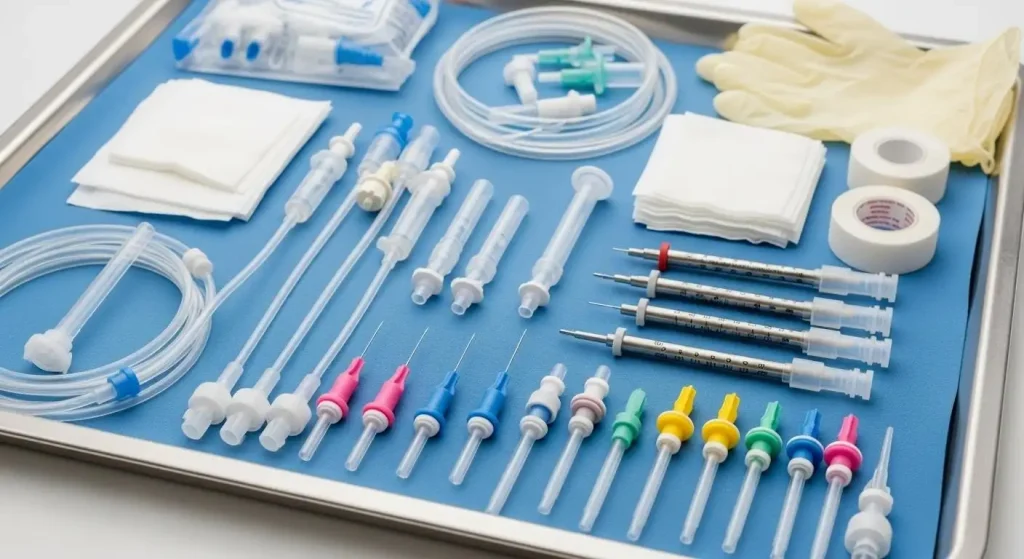
1) Regulatory Evidence You Can Verify Quickly
Compliance should be demonstrable, current, and mapped to the exact devices you intend to buy. An IV sets and cannulas supplier needs more than logos on a slide deck; they must provide traceable, auditable documentation with valid dates and scopes.
Request and read through:
• ISO 13485 certification whose scope explicitly covers IV sets, cannulas, syringes, and hypodermic accessories
• FDA establishment registration plus product listings for the relevant device categories
• CE certificates and Declarations of Conformity tied to named product families and current standards
• Sterilization validations (method, SAL), bioburden and endotoxin data, and representative batch release records
Greetmed, established in 2003, operates under an ISO 13485 quality management system and maintains FDA and CE credentials. These frameworks anchor design controls, risk management, sterility assurance, and post-market surveillance. Verified compliance reduces nonconformities, accelerates market registrations, strengthens tenders, and simplifies audits. A compliant IV sets and cannulas supplier protects your patients and your brand while making your quality and regulatory teams more effective.
2) Clinical Performance That Holds Up in Real Use
Small design flaws can erode care: drip rates that drift and complicate titration, tubing that kinks during patient movement, microleaks at Luer interfaces, or cannulas that dislodge under routine repositioning. Your IV sets and cannulas supplier should show that their devices deliver stable therapy and safer handling where it matters - on the ward, in the ED, and in ICU.
Look for design choices and data that translate into measurable outcomes:
• Anti-kink tubing that preserves lumen integrity under flex and secure Luer connections that resist microleaks after repeated manipulations
• Spike and drip chamber geometry that support quick, bubble-free priming and clear visibility for monitoring
• Cannula tip and catheter flexibility that enable atraumatic insertion and a secure, comfortable dwell
• Safety features such as clips or auto-disable mechanisms that reduce needlestick risk and discourage reuse
Greetmed engineers infusion and transfusion sets for precise delivery and leak resistance, and cannulas for smooth venipuncture with firm fixation. The hypodermic range - needles, syringes, safety caps, and hubs - prioritizes sterility and predictable handling. Safety and auto-disable syringe options support infection control by limiting reuse. Beyond brochures, Greetmed backs claims with validation reports, use-phase testing, and post-market surveillance that show device performance across routine and demanding clinical scenarios.
3) Traceability That Accelerates Investigations
When a question arises - about sterility, a component, or a reported event - traceability is your shortest route to a clear answer. If lot control stops at finished goods or complaint records are fragmented, investigations drag on and liability grows. A dependable IV sets and cannulas supplier should make it straightforward to reconstruct device history from raw material to shipment.
Expect tangible proof of end-to-end traceability:
• Lot-level records linking incoming raw materials, in-process controls, sterilization parameters and release, and outbound shipping
• A structured complaint-handling process, with trend analysis and corrective actions (CAPAs) available for customer review
• Formal change control with timely notifications for material, tooling, or process changes that could impact performance
• Audit readiness, including access to quality manuals, procedures, and sample Device History Records during supplier audits
Greetmed maintains full lot traceability and communicates changes with transparency. That rigor shortens root-cause analysis, narrows any recall scope to affected batches, and leaves cleaner audit trails. Day to day, it reduces uncertainty, speeds responses to clinicians, and reinforces patient safety.
4) Supply Reliability That Keeps Care Continuous
Clinical demand does not pause for upstream hiccups. Shortages lead to substitutions, retraining, and workflow adjustments that heighten risk and cost. Verify that your IV sets and cannulas supplier can perform consistently through seasonal highs, tender shocks, and public health emergencies.
Probe resilience with concrete indicators:
• Quantified capacity and dependable lead times by product family: infusion sets, transfusion sets, cannulas, syringes, and lancets
• Safety stock thresholds and surge protocols aligned to emergency procurement and tender volumes
• Transparent logistics with on-time performance metrics, proactive delay alerts, and corrective action plans
• Continuity measures, including dual sourcing where possible and rapid lot replacement mechanisms
Greetmed plans capacity and keeps safety stock to support hospitals and distributors at scale, harmonizing forecasts with epidemiologic trends and tender schedules. A reliable IV sets and cannulas supplier minimizes clinical downtime, curbs emergency procurement premiums, and reduces training burdens caused by forced product switches - producing steadier care with fewer operational surprises.
5) A Coherent Portfolio That Reduces Friction
On paper, mixing brands can look flexible. In practice, it increases incompatibilities, training variance, and documentation overhead. Using a single supplier for IV sets and cannulas reduces portfolio complexity, enables standardized practice, and strengthens quality control.
Greetmed's portfolio spans:
• Infusion therapy: IV infusion sets (including flow-controlled choices) with clear drip chambers, kink-resistant tubing, and secure connectors
• Transfusion therapy: blood transfusion sets with in-line filtration and reinforced spikes for safe administration
• Vascular access: IV cannulas designed for smooth insertion, reliable dwell, and secure fixation
• Hypodermics and accessories: disposable syringes, U-100 insulin syringes, insulin pen needles, safety clip syringes, auto self-destruct syringes, and lancets (plastic-handle and pressure-activated)
• One supplier improves compatibility, aligns quality systems, lightens training, and simplifies post-market surveillance. It gives you one accountable partner across infusion, transfusion, and hypodermic workflows.
6) Partnership, Support, and Continuous Improvement
Consistent performance requires responsive service and a closed feedback loop that enables continuous improvement. An accountable IV sets and cannulas partner remains involved across implementation, validation, and lifecycle management to avoid patient-side gaps.
Translate partnership into daily wins:
• Bid, regulatory, and committee guidance delivered with complete technical files
• Role-specific training and onboarding materials for priming, insertion, securement, and disposal aligned with your SOPs
• Recurring joint quality and business reviews to follow KPIs, detect patterns, and plan upgrades
• Clear, transparent complaint processes with agreed SLAs and documented corrective and preventive actions
Greetmed supplies comprehensive technical files, clinician evaluation kits, and onboarding built around nursing workflows. Field feedback is captured and channeled into design and process changes so improvements stick and scale. A partner-first IV sets and cannulas supplier lowers total cost of ownership while safeguarding compliance and safety throughout the device lifecycle.
7) Common Pitfalls When Vetting Suppliers
Even seasoned teams can overlook early signals that later become operational issues. Watch for:
• Certification scope errors, expired certificates, or no demonstrable link to your product families
• Assertions lacking validated test data, usability findings, or post-market performance metrics
• Traceability truncated at finished goods with absent raw-material and sterilization documentation
• Capacity claims that lack specifics, no surge coverage, and erratic delivery indicators
• Product portfolios too narrow, driving multi-vendor dependence and training complexity
• Complaint handling that's delayed and nontransparent, leaving clinicians waiting
A methodical review of these areas helps you differentiate shiny decks from operationally sound suppliers.
Next Steps
If you are reassessing your IV sets and cannulas supplier, speak with Greetmed. Request our compliance dossier, lot traceability overview, and a comprehensive sample kit spanning infusion, transfusion, cannulas, syringes, and lancets. Our team will align product fit with your clinical protocols and help de-risk supply, audit readiness, and bedside performance.
Choosing well is more than procurement; it's a commitment to patient safety and organizational resilience. Greetmed stands ready with certified quality, proven performance, and a portfolio that simplifies care - so your clinicians can focus on what matters most.
Submit Your Request
Recent Posts
Tags
- Adult Diapers
- Are custom medical devices safe
- Baby Diapers
- Can respiratory anesthesia be used
- Digital Healthcare
- Do you offer customized consumables
- European Market
- How do you take care of a skin wound
- Industry Trends
- Lady Sanitary Napkins
- Medical Devices
- OEM Medical Devices
- Product Introductions
- Protective Equipment
- Under Pads
- What are custom-made medical devices
- What are diagnostic products
- What are hospital dressing products
- What are medical tube catheters
- What are some common protective equipment
- What are the appropriate applications for hospital dressing products
- What are the appropriate uses for protective equipment
- What is a gynecological examination
- What is a medical consumable
- What is an anesthesia kit
- What is an OEM in medical devices
- what is an wound skin care
- what is can disposable ultrasonic diagnostic
- What is good manufacturing medical devices
- What is hospital-grade protective equipment
- what is medical equipments hospital furniture
- What is medical sterilization wrapping
- What is rehabilitation equipment device
- What medical consumables do you supply
- Where can I find laboratory consumables wholesale
- where can I find medical protection device
- where to buy hypodermic accessories
- where to buy medical apparel
- where to buy medical consumable accessories
- where to find OEM medical device supplier
- where to find rehabilitation equipment supplier