Anesthesia Equipment Kit 2026: How to Pick Safe, User-Friendly Supplies
Anesthesia Equipment Kit selection in 2026 demands clear, evidence-based choices. This guide will help you master the following: safety-focused design, core components, age- and trauma-specific configurations, infection control with single-use solutions, and practical supplier evaluation.
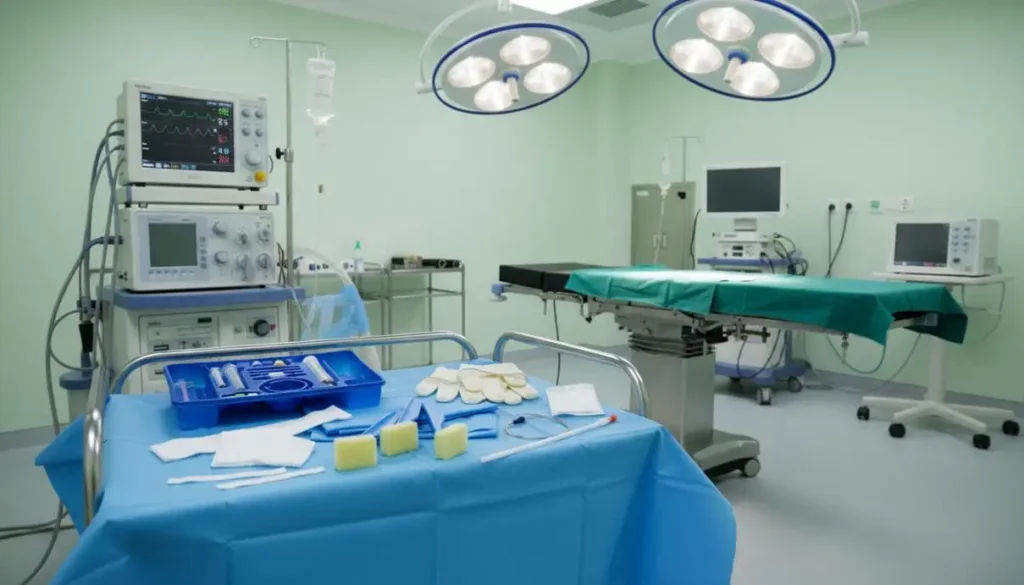
Safety and Usability: Design That Prevents Error
When the room is busy and time is limited, human-factor design is not optional. An effective Anesthesia Equipment Kit uses intuitive compartmentalization, color-coded labels, and clear typography. Each item is accessible without visual clutter. Tactile differences between components reduce the risk of picking the wrong device under gloves. Peel-open sterile wrappers minimize handling steps and keep the field clean.
These features prevent delay and reduce cognitive load during rapid-sequence intubation, emergency airway management, and trauma care. They also support team communication: clear labels and standardized placement make verbal requests precise and fast. Evidence from the WHO Surgical Safety Checklist shows that structured processes can lower complications by roughly 30 - 40%. A kit that reinforces standardized steps brings the same discipline into equipment handling, where many small errors originate.
What's Inside an Anesthesia Equipment Kit
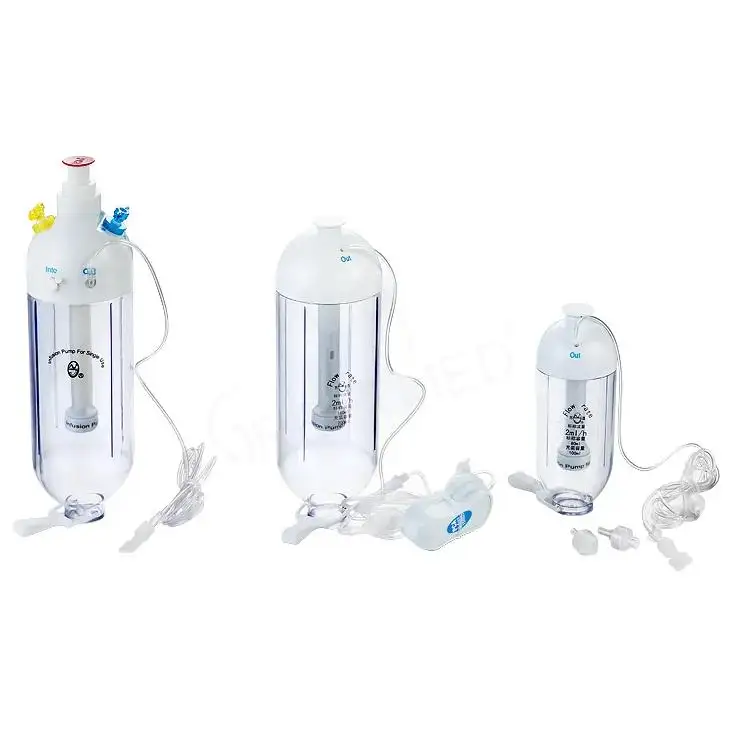
A modern Anesthesia Equipment Kit is a pre-assembled set of essential tools for safe anesthesia delivery. Contents are organized and sterile, allowing teams to begin immediately. A typical kit includes a face mask, syringes, IV cannulas, airway devices, antiseptic swabs, gloves, and adhesive tape. Depending on clinical needs, it can also include endotracheal tubes, supraglottic airway devices, laryngoscope blades, and oxygen delivery components.
Pre-packing serves two goals. First, it shortens setup time and reduces missing-item risk. Second, it ensures case-to-case consistency. Clinicians devote time to assessment and anesthesia planning instead of tracking down supplies. This reliability is critical in hospitals, surgical centers, emergency departments, ambulatory settings, and field environments where time and sterility are decisive.
Configurations for Adult, Pediatric, Neonatal, and Trauma Care
No single layout fits every patient. A well-structured anesthesia kit portfolio includes adult, pediatric, and neonatal variants, each sized and selected to match patient physiology for consistent care. Dedicated trauma kits round out the range with a focus on rapid access, effective bleeding control, and timely stabilization. Dedicated lines support GA and regional/local techniques. Custom configurations reflect hospital preferences, formulary limits, and training design - fewer surprises, faster onboarding.
Broad coverage curbs variability. Mixed sources and improvised sets lead to inconsistent packaging, unfamiliar devices, and missing accessories. Standardized kits reduce variation, accelerate orientation of new staff, and enhance cross-cover capability between units.
Infection Control: Why Single-Use Matters
Disposable, single-use kits are now standard in many settings. Sterile, one-time use reduces cross-contamination risk and removes the burden of reprocessing. The CDC estimates that 1 in 31 hospitalized patients in the United States has a healthcare-associated infection on any given day. WHO reports that up to half of such infections are preventable. Single-use Anesthesia Equipment Kit design contributes to prevention by taking contaminated reusables out of the equation, especially for high-touch airway supplies.
Single-use also improves workflow. There is no need to track returns to central processing or to manage turnaround delays. Consistency from case to case limits variability in device performance. For busy teams, this stability supports predictable schedules and less downtime. To address sustainability, look for kits with recyclable outer packaging and clear waste-stream guidance, so compliance and environmental goals can coexist.

Selection Criteria and Supplier Evaluation Checklist
Choosing the right partner requires a structured review. Use the checklist below to align safety, usability, and supply continuity with your clinical goals.
✓ Clinical Safety Features: Color-coded labels, intuitive layout, clear IFUs, and sterility assurance for each component
✓ Patient Coverage: Adult, pediatric, neonatal, and trauma configurations with appropriate sizing and airway options
✓ Infection Control: Single-use design, sealed packaging integrity, and evidence of validated sterile processes
✓ Traceability: Lot codes and documentation for auditing, recall readiness, and quality improvement
✓ Standards And Compliance: Alignment with recognized quality systems and regulatory requirements
✓ Training And Onboarding: Visual guides, competency materials, and in-service support for fast adoption
✓ Supply Continuity: Stable lead times, buffer stocks, and contingency plans for peak demand or disruptions
✓ Total Cost Of Ownership: Transparent pricing, reduced setup time, and elimination of reprocessing costs
✓ Sustainability: Recyclable packaging elements and waste-segregation instructions
✓ Customization: Ability to match protocol preferences and integrate formulary choices without delayed fulfillment
Our Solutions: Turning Features into Practical Gains
We supply a comprehensive range of disposable medical items - gloves, masks, syringes, urine bags, and more - alongside the Anesthesia Equipment Kit. For clinicians, this single-source approach reduces stock fragmentation and lowers the risk of substitutions. Sterile, pre-packed kits convert setup into a straightforward workflow, reducing downtime and enabling prompt starts. Color-coded compartments aid fast identification when pressure is high. Dedicated age and trauma variants keep the right device within reach, lowering event risk and enhancing continuity between units.
Management benefits include consistent product quality with lot traceability for stronger compliance and quicker audits. Centralized procurement simplifies vendor management and stabilizes inventory. The removal of reprocessing lowers hidden labor and variability in device condition. Clear IFUs and structured in-service training accelerate onboarding for rotating staff.
Call to Action: Build a Safer, Smarter Anesthesia Equipment Kit Program
If your goals include fewer setup delays, stronger infection control, and better human-factor design, it is time to standardize. Request a sample Anesthesia Equipment Kit, schedule a clinical evaluation with your anesthesia leads, or ask for a configuration aligned to your protocols. Our team will help you test usability, verify patient coverage, and map the supply plan that keeps your procedures safe, efficient, and consistent.
Submit Your Request
Recent Posts
Tags
- Adult Diapers
- Are custom medical devices safe
- Baby Diapers
- Can respiratory anesthesia be used
- Digital Healthcare
- Do you offer customized consumables
- European Market
- How do you take care of a skin wound
- Industry Trends
- Lady Sanitary Napkins
- Medical Devices
- OEM Medical Devices
- Product Introductions
- Protective Equipment
- Under Pads
- What are custom-made medical devices
- What are diagnostic products
- What are hospital dressing products
- What are medical tube catheters
- What are some common protective equipment
- What are the appropriate applications for hospital dressing products
- What are the appropriate uses for protective equipment
- What is a gynecological examination
- What is a medical consumable
- What is an anesthesia kit
- What is an OEM in medical devices
- what is an wound skin care
- what is can disposable ultrasonic diagnostic
- What is good manufacturing medical devices
- What is hospital-grade protective equipment
- what is medical equipments hospital furniture
- What is medical sterilization wrapping
- What is rehabilitation equipment device
- What medical consumables do you supply
- Where can I find laboratory consumables wholesale
- where can I find medical protection device
- where to buy hypodermic accessories
- where to buy medical apparel
- where to buy medical consumable accessories
- where to find OEM medical device supplier
- where to find rehabilitation equipment supplier